Having problems reading this email? View it in your browser >>
|
||
 |
||
|
||
Contents
|
||
Clinics will need new systems to administer injectable HIV therapies | ||
 Dr Jonathan Angel presenting to EACS 2021. | ||
|
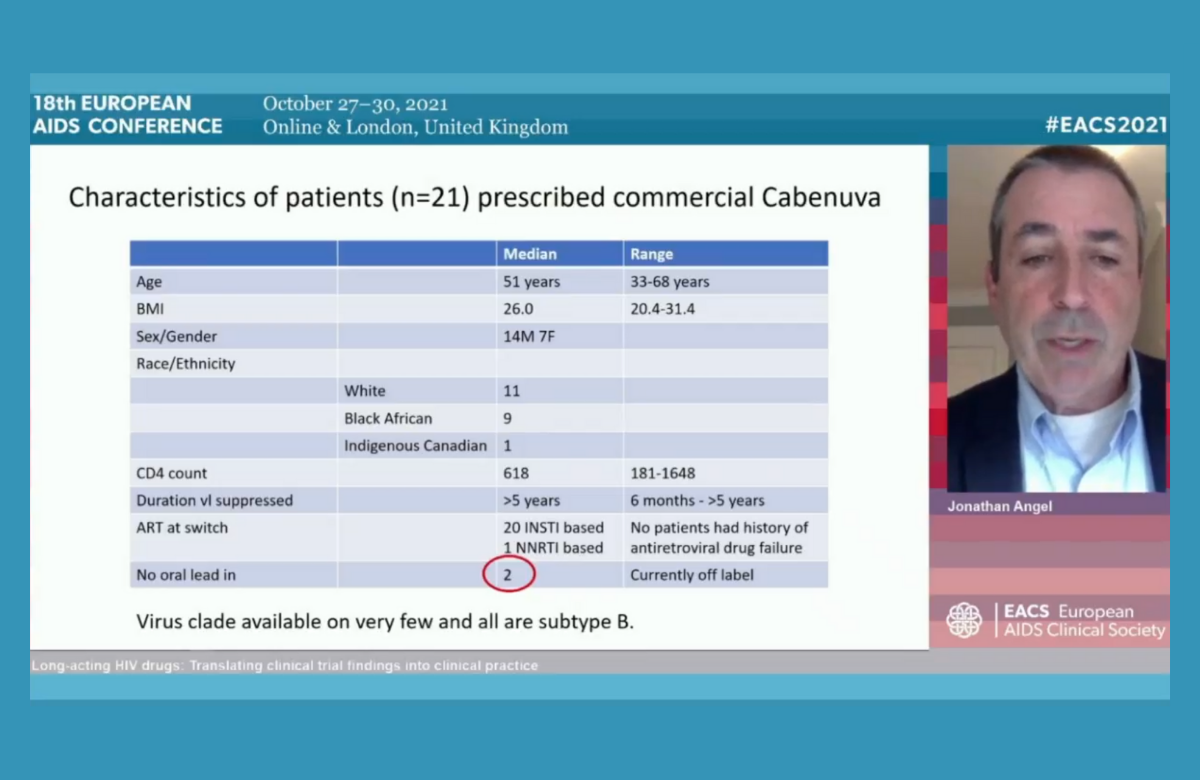
Dr Jonathan Angel of the University Hospital of Ottawa in Canada told the 18th European AIDS Conference (EACS 2021) last week about his practical experience of prescribing the new injectable antiretrovirals cabotegravir and rilpivirine. In addition to five years’ experience of administering them as part of the clinical trials, he is one of the first doctors to provide them in routine care. Canada was the first country to license the long-acting antiretroviral treatment, which is sold in North America under the brand name Cabenuva. In Europe, the two drug formulations have the separate brand names of Vocabria and Rekambys. Dr Angel said that administering injections every month or two to a significant number of patients would be an extra burden on any busy outpatients’ department. Provision in Canada has been facilitated by the existence of a pharmaceutical company sponsored scheme called Cabenuva Supports which actually administers the injections. The injections are provided by nurses off-site, in primary care clinics, pharmacies or in people’s homes. Dr Angel said that he had found it impossible to predict which patients would express a serious interest in switching to injectable therapy: apart from simply preferring injections, their reasons were very individual and often multiple. “When made aware of injectable therapy, patients will select themselves,” he said. Most patients have chosen to have an oral ‘lead-in’ of four weeks of cabotegravir and rilpivirine pills, but some data suggest that it may not be necessary and a few patients have chosen not to have an oral lead-in. A handful of patients have decided to discontinue the injections and go back on to oral therapy. This was mostly due to ‘injection fatigue’ – the inconvenience of arranging injection appointments and side effects. One interesting possible use of the injectables would be as temporary antiretroviral therapy during periods when oral pills were inconvenient or might raise concerns about unwanted disclosure of HIV status, for example when travelling. “We are just at the beginning of using these technologies,” Dr Angel commented, “and we will come across other clinical and practical issues as more patients use them. They won’t necessarily solve ongoing poor adherence, because patients can miss injections too. But there is no doubt a large proportion of patients will want them.” | ||
Case report of long-lasting SARS-CoV-2 shedding in immunocompromised patient | ||
 Dr Irfaan Maan presenting to EACS 2021. | ||
|
People with HIV who are severely immunosuppressed may have prolonged periods of shedding SARS-CoV-2 (the virus which causes COVID-19) without clinical symptoms, a case report presented to the conference suggests. Dr Irfaan Maan of University College London described the case of a 28-year-old woman with HIV who was diagnosed with B-cell lymphoma (a form of the cancer non-Hodgkin lymphoma) in mid-2020, with a CD4 count of 30 and a viral load of 354,814 copies/ml. She had a long history of suboptimal antiretroviral adherence and her lymphoma diagnosis came after a 10-month treatment break. Upon re-engaging with care, she resumed antiretroviral therapy and commenced chemotherapy, both with good long-term results. However, her CD4 count remained low for several months and she tested positive for SARS-CoV-2 on a PCR test. Unusually, she continued to test positive at day 15, again at days 22 and 37, and remained positive on PCR and transcription mediated amplification (TMA) assays until 164 days after her first positive test. An antibody test at day 64 was negative, suggesting a lack of immune response to infection. Throughout the time she tested positive, she had no COVID-19 symptoms. Prolonged viral shedding has been previously reported in people with severe immunosuppression, including another person with HIV. However, it is unclear whether the viral shedding is sufficient to pose a risk of infection to others and in this case, two family members living in the same house were not infected. The patient was forced to self-isolate for over five months, which had a significant psychosocial impact on her. She missed social contact, and experienced low mood and frustration with the situation. She needed support from clinicians and clinic psychologists to improve her mood and outlook on her situation. The case highlights the need to understand more about SARS-CoV-2 and COVID-19 in immunosuppressed people, not just vaccination responses. | ||
Most people with HIV accept COVID-19 vaccination | ||
 Gustavo Fring/Pexels | ||
|
Surveys from diverse settings do not suggest unusual levels of COVID-19 vaccine hesitancy among people living with HIV, the conference heard last week. The largest of the surveys was of 1486 people living with HIV in Argentina: 84% said they would have the vaccine if recommended by a healthcare provider and 79% if the government mandated it. Safety was a crucial factor for those who were hesitant about vaccination. Broadly similar results came from studies in Greece (81% already vaccinated or willing), Turkey (70%), the Middle East (65%) and young people in the United Kingdom (75%). There were few demographic factors associated with vaccine hesitancy, but included female gender, lower levels of education and living outside a major city in the various studies. The positive impact of HIV clinicians’ discussing and recommending COVID-19 vaccines was a recurrent theme. Moreover, as perceived safety appears to be the main driver of vaccine acceptability, educating people living with HIV about the available safety data in this specific population is likely to increase vaccine acceptability. | ||
Europe far from achieving hepatitis C elimination targets | ||
 Dr Erika Duffell presenting to EACS 2021. | ||
|
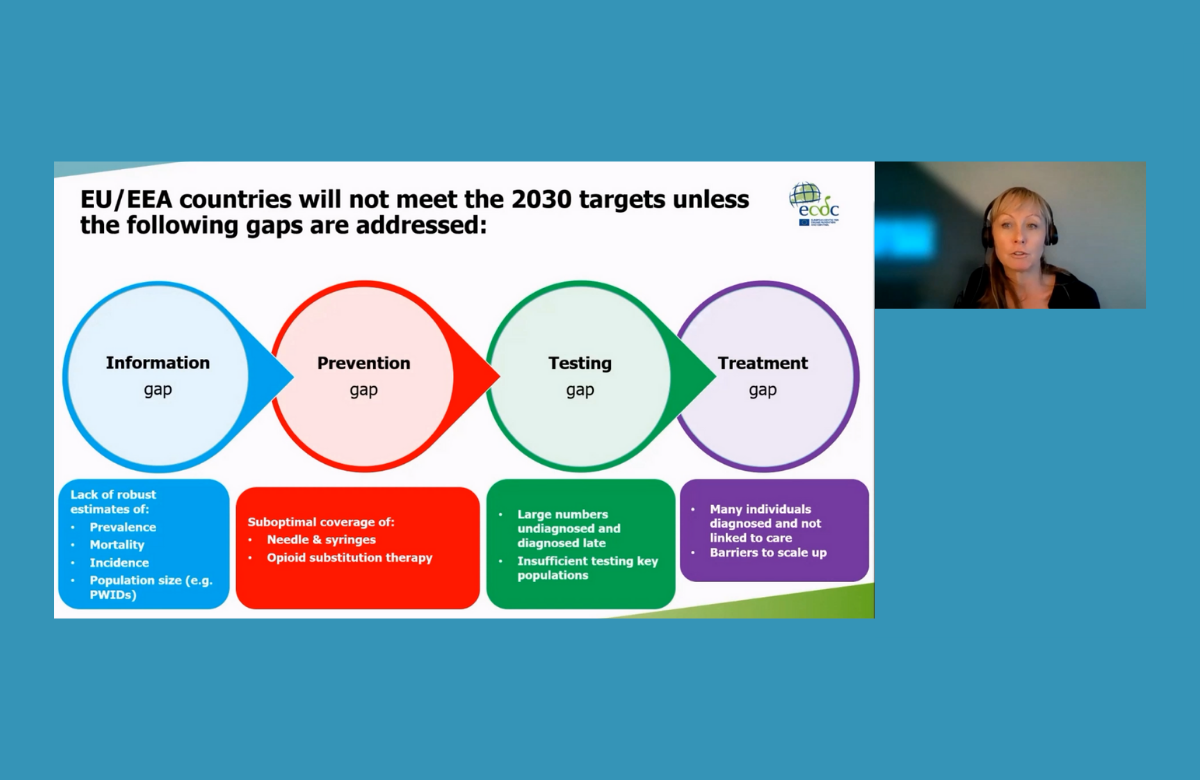
European Union states are far from reaching the global targets towards hepatitis C elimination, especially on harm reduction, testing and treatment, Dr Erika Duffell of the European Centre for Disease Control told the conference. Global targets were agreed in 2016 in order to reduce new viral hepatitis infections by 90% and reduce deaths due to viral hepatitis by 65% by 2030. Dr Duffell reviewed progress on elimination of hepatitis C, the predominant hepatitis virus in the European region. However, the majority of countries in the region do not have up-to-date estimates of how many people are living with hepatitis C, the number of recent diagnoses, or how many people treated for hepatitis C achieve a sustained virologic response. Harm reduction coverage remains weak. By 2019, only three countries had achieved the target of distributing 200 syringes per drug user per year. Only nine countries reported that they had hit the target of providing opioid substitution therapy to 40% of high-risk opioid users. Only four countries in the region – France, Ireland, Italy and Sweden – were estimated to have diagnosed more than half of people living with hepatitis C by 2020. The prevalence of undiagnosed cases is especially high in Romania and Greece. “COVID has shown what can be done on testing at a large scale and we need to channel some of that innovation into testing for hepatitis C,” said Dr Duffell. | ||
PrEP implementation remains limited in central and eastern Europe | ||
 Dr Justyna Kowalska presenting to EACS 2021. | ||
|
Oral pre-exposure prophylaxis (PrEP) is still nowhere nearly available enough in the parts of Europe that need it most, Dr Justyna Kowalska of the Medical University of Warsaw told the conference. Even the country providing PrEP to more people than any other in central and eastern Europe – Ukraine, with just under 4000 people starting PrEP since 2018 – would be supplying PrEP to 62,500 people, if the size of its PrEP programme relative to its population of people living with HIV was the same as that in France or the UK. While Poland also has a relatively large programme, provision in countries such as Slovenia, Croatia, Czechia, Georgia and Moldova is on a small scale. In six countries in the region there are no plans as yet to even license PrEP. There are institutional barriers to the scale-up of PrEP, such as national healthcare plans not paying for it; national guidelines not recommending it; and the medication not being formally licensed in a country, meaning that if doctors do prescribe it, it is 'off-label' and the doctor would be liable for any adverse consequences. High levels of interest in PrEP in Poland and Ukraine are partly due to PrEP leading to the creation of a network of community-friendly sexual health clinics. These have resulted in greater engagement by many gay and bisexual men with their sexual health in a broader sense. Elsewhere, most PrEP services and service providers remain highly medicalised, but a greater diversity of service delivery models could help engage more people with PrEP. | ||
aidsmapLIVE: HIV prevention special
On Monday 8 November at 6pm (UK time), NAM aidsmap is broadcasting an aidsmapLIVE HIV prevention special. NAM aidsmap’s Susan Cole will be joined by guests Professor Sheena McCormack from the Medical Research Council Clinical Trials Unit; Dr Vanessa Apea from Barts Health NHS Trust; Winifred Ikilai from the National Forum of People Living with HIV/AIDS Networks in Uganda; and Gus Cairns from NAM aidsmap. You can watch the broadcast on our Facebook and Twitter pages. | ||
Connect with us |
||
|
NAM's news coverage of the 18th European AIDS Conference has been supported by the European AIDS Clinical Society, Gilead Sciences Europe Ltd and ViiV Healthcare. |
||
|
aidsmap is an award-winning, community-based organisation, which works from the UK. We deliver reliable and accurate HIV information across the world to HIV-positive people and to the professionals who treat, support and care for them.
NAM Publications
Cally Yard, 439 Caledonian Road, London N7 9BG Company limited by guarantee. Registered in England & Wales, number: 2707596 Registered charity, number: 1011220 To unsubscribe please click here Privacy Policy: www.aidsmap.com/about-us/confidentiality |