Having problems reading this email? View it in your browser >>
|
||
 |
||
|
||
Contents
|
||
Earlier treatment and higher CD4 counts linked to smaller HIV reservoir | ||
 Dr Brian Moldt presenting to IAS 2021. | ||
|
The results were presented to the 11th International AIDS Society Conference on HIV Science (IAS 2021). Very soon after initial infection, HIV establishes a lasting reservoir of inactive virus in long-lived resting T-cells. While antiretroviral medications can control viral replication, they do not eliminate these latent viral blueprints, which can resume virus production when treatment stops – the key barrier to a cure for HIV. In one study, researchers compared the size of the viral reservoir in people who started antiretroviral therapy (ART) with a CD4 count of 500-599, 600-799 or more than 800. After three years on treatment, total HIV DNA was lower in people who started ART with a CD4 count above 800. In another study, a research team compared viral reservoir size and diversity, as well as HIV's susceptibility to broadly neutralising antibodies (bNAbs), in people who started ART at different stages of infection. bNAbs are currently being studied in HIV treatment, prevention and cure research. The study enrolled people in four cohorts based on when they started ART: Fiebig stage I or II (when HIV RNA and the p24 antigen can be detected), Fiebig III or IV (when HIV antibodies are first detectable), late acute infection (three months or less) and chronic infection (six months or less). Participants had been on treatment for three to five years and CD4 counts were high (700 to 900). The researchers found that total DNA was lower in the Fiebig I-II and Fiebig III-IV groups and viral diversity was lower in people who started treatment during acute infection compared with chronic infection. People who started treatment earlier also generally had greater susceptibility to the bNAb medication elipovimab, currently in development. A third study investigated early ART and post-treatment control – the ability to maintain viral suppression after stopping therapy – in 36 macaque monkeys with SIV, the equivalent of HIV. ART was started at various time points, was continued for two years, then the monkeys were monitored for six months to a year after treatment interruption. Viral rebound (defined as more than 1000 copies/ml) occurred later in those that started treatment within 28 days of exposure compared with those starting treatment after six months. What's more, 82% of the monkeys in the early ART group achieved post-treatment control (defined as a viral load below 400) after treatment interruption, compared with 25% in the second group. | ||
Shorter, less toxic treatment for highly drug-resistant TB cures nine out of ten | ||
 Dr Francesca Conradie (top right) at IAS 2021. | ||
|
A six-month oral treatment regimen that limits exposure to the toxic drug linezolid can achieve a high cure rate in people with highly drug-resistant tuberculosis (TB), IAS 2021 heard this week. Eighty-nine per cent of study participants remained TB-free six months after completing treatment. Most recommended regimens for treatment of multidrug-resistant TB (MDR-TB) must be taken for at least nine months and some for up to 20 months. In 2020, a study showed that highly drug-resistant TB could be treated successfully with a six-month oral course of bedaquiline, pretomanid and linezolid. However, study participants had a high frequency of serious side effects due to linezolid. In the study reported this week, participants received either a reduced dosage or a shorter duration of linezolid treatment. All received bedaquiline and pretomanid, and they were randomised to receive one of four linezolid treatment courses: 1200mg daily for 6 months; 1200mg daily for 2 months; 600mg daily for 6 months; or 600mg daily for 2 months. The study recruited people with extensively drug-resistant TB (41%), people with MDR-TB that showed resistance to either a fluoroquinolone or an injectable TB drug (47%), people who had failed to respond to at least six months of standard MDR-TB treatment (6%) and people with rifamycin-resistant TB unable to tolerate drugs in the standard MDR-TB treatment regimen (5%). The study recruited 181 participants in Georgia, Moldova, Russia and South Africa. Participants were predominantly male (67%), 36% were Black and 20% were living with HIV. There was no significant difference in treatment failure: there were favourable outcomes for 93%, 88%, 90% and 84% in the respective study arms, an average of 89%. The frequency of serious adverse events was lowest in the group taking 600mg linezolid for two months. Dr Francesca Conradie said, “The results of this study are very reassuring. With a reduction in the dose and/or duration of linezolid, we can still offer patients a high chance of cure in only six months.” A second study of a six-month oral treatment regimen for MDR-TB was halted in March because the experimental regimen showed superiority to the standard-of-care control treatment. The results of the study of a regimen consisting of bedaquiline, pretomanid, linezolid 600mg and moxifloxacin 400mg are likely to be published later this year. Trial sponsor Médecins Sans Frontières hopes the World Health Organization will change its guidance on MDR-TB treatment after reviewing the results. | ||
Modelling suggests injectable cabotegravir is far more effective than daily oral PrEP for women | ||
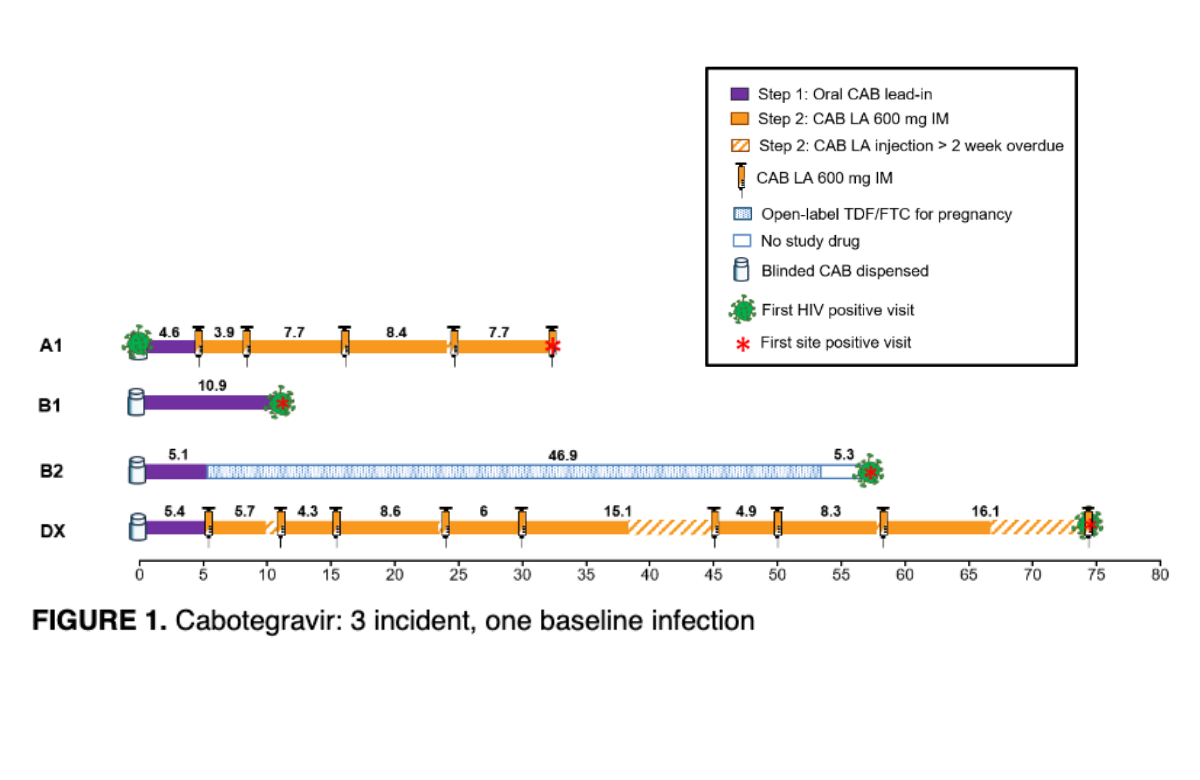 Diagram from Dr Sinead Delany-Moretlwe's e-poster presentation. | ||
|
The HPTN 084 trial compared the safety and efficacy of PrEP (regular medication to prevent HIV infection) given as two-monthly injections of cabotegravir versus daily oral PrEP using tenofovir disoproxil fumarate and emtricitabine (TDF/FTC) in more than 3000 sexually active cisgender women between the ages of 18 and 45 in seven countries in sub-Saharan Africa. The blinded phase of the trial was halted in November 2020 after interim results showed that women randomly assigned to use cabotegravir injections had an 89% lower risk of HIV acquisition compared with those using daily TDF/FTC pills. This is the highest efficacy ever seen in a trial of PrEP for women. While daily oral PrEP has had excellent results in men who have sex with men, studies have found it to be less effective for women – largely due to suboptimal adherence. There were just four new infections among women randomly assigned to cabotegravir compared with 36 among those assigned to TDF/FTC. A new analysis has looked at these in more detail and found that missed doses was probably the cause in all but two of the infections. A separate mathematical modelling study compared new HIV infections among women using injectable cabotegravir in HPTN 084 against a hypothetical placebo control derived from previous studies. This provides an estimate of the expected incidence of new HIV infections if no biomedical prevention method was used. The researchers used placebo group data from a previous trial, which tested TDF/FTC pills and a tenofovir vaginal gel. This projected a placebo incidence of 2.2% for the HPTN 084 study cohort. Comparing this against the incidence rate of 0.2% in the cabotegravir arm, the researchers estimated that injectable cabotegravir is 91% effective at preventing HIV versus a placebo. | ||
An HIV prevention research agenda for transgender and gender diverse people | ||
 JD Davids at the 'No Data No More' press conference. | ||
|
There are an estimated 25 million transgender adults worldwide, with a global HIV prevalence of 19% among transgender women – this is 49 times higher than that of the general population. Between 2010 and 2019, global HIV incidence rates in trans women increased by 5%, widely missing UNAIDS’ target to decrease new HIV infections by 75% in this key population by 2020. There is a scarcity of HIV data on trans men, while gender nonbinary people, who do not necessarily identify with any particular gender, are further lost in the statistics. The authors of the manifesto highlight the need for a better understanding of contributors to HIV incidence, demanding that, “Key drivers of transgender HIV incidence are comprehensively identified – including biomedical, social and structural factors across countries and regions.” They call for HIV prevention studies to improve their inclusion of transgender and gender diverse people, so as to enable analysis of results between different subgroups, and to centre the concerns of trans people in HIV prevention research. The authors highlight the limited number of studies that currently adhere to this vision. The authors describe gender-affirming hormone therapy as a crucial aspect of the health and wellbeing of many trans people and emphasise the need for interactions between hormone therapy and HIV drugs to be more thoroughly researched. A central theme throughout the No Data No More manifesto is the need for trans leadership and partnership. Co-author, Immaculate Mugo, said, “For trans-focused programs to be effective, trans people – who recognise the subtleties and nuances that often go overlooked – need to be in the leadership seat, informing in-depth analyses and recommendations to ultimately shift the way research informs policy and programs.” | ||
aidsmapLIVE: IAS 2021 special
On Monday 26th July at 6pm (BST), NAM aidsmap is broadcasting an aidsmapLIVE IAS 2021 special on its Facebook and Twitter pages. Susan Cole from NAM aidsmap will be discussing news and research presented at IAS 2021 with guests: Dr Meg Doherty, Director of Global HIV, Hepatitis and STI Programmes at the World Health Organization; Dr Laura Waters, Chair of the British HIV Association; Phelister Abdalla, National Co-ordinator of the Kenya Sex Workers Alliance; and Dr Christoph Spinner, IAS 2021 rapporteur and Chief Medical Information Officer at the University Hospital Rechts der Isar, Germany. | ||
Scientific analysis from Clinical Care Options
Clinical Care Options is the official provider of online scientific analysis for the IAS 2021 conference through capsule summaries, downloadable slides, rapid expert webinars, and ClinicalThought commentaries. Clinical Care Options will be hosting three live, interactive, webinars for HIV healthcare professionals on 22nd and 23rd July. Professor Chloe Orkin (22nd at 1pm ET), Dr Daniel R. Kuritzkes (22nd at 4pm ET) and Professor Babafemi Taiwo (23rd at 1pm ET) will provide a rapid update from the recent IAS scientific meeting and answer questions. | ||
Connect with us |
||
Official conference partners |
||
|
NAM's news coverage of IAS 2021 has been made possible thanks to support from Gilead Sciences Europe Ltd and ViiV Healthcare. |
||
|
aidsmap is an award-winning, community-based organisation, which works from the UK. We deliver reliable and accurate HIV information across the world to HIV-positive people and to the professionals who treat, support and care for them.
NAM Publications
Cally Yard, 439 Caledonian Road, London N7 9BG Company limited by guarantee. Registered in England & Wales, number: 2707596 Registered charity, number: 1011220 To unsubscribe please click here Privacy Policy: www.aidsmap.com/about-us/confidentiality |