Fostemsavir, a new experimental attachment inhibitor, suppressed viral load in over half of participants with extensive drug resistance when added to a background regimen selected by resistance testing, Max Lataillade of ViiV Healthcare reported at the 16th European AIDS Conference (EACS 2017) in Milan on Friday.
The findings come from the phase 3 BRIGHTE study carried out in the United States, France and Brazil.
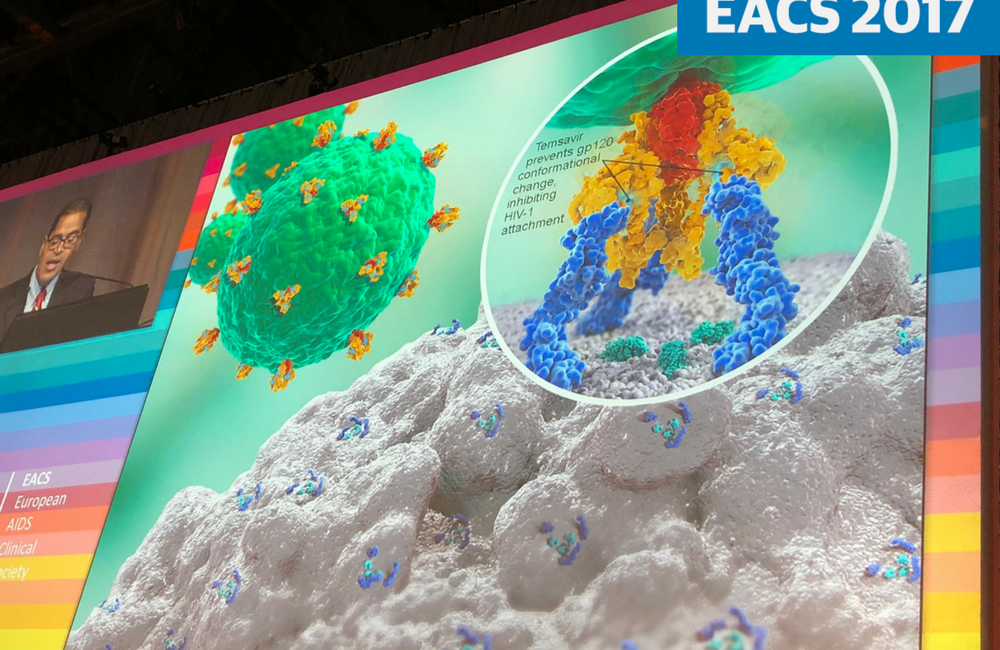
Fostemsavir (formerly BMS-663068) is a new experimental HIV attachment inhibitor which binds to the HIV gp120 protein, preventing HIV attachment to CD4 cells. Other inhibitors of HIV entry, enfuvirtide and maraviroc, have limited roles in HIV treatment. Enfuvirtide is an HIV fusion inhibitor, an injectable agent that is prescribed only for people with no other treatment options. Maraviroc is a CCR5 antagonist; it prevents HIV from using the CCR5 receptor on the surface of CD4 cells to gain entry to the cell. It is used in treatment-experienced people.
Fostemsavir is being developed by ViiV Healthcare as an agent for use in treatment-experienced people with resistance to several classes of antiretroviral drug. The drug was acquired from Bristol-Myers Squibb along with several other experimental antiretroviral drugs in 2016.
Clinical trials of fostemsavir have tested the drug in people with HIV who have two or fewer active classes of antiretroviral drug available to them.
A substantial minority of people living with HIV who began treatment in the mid-1990s have been exposed to suboptimal regimens and have developed resistance to non-nucleoside reverse transcriptase inhibitors, nucleoside reverse transcriptase inhibitors and most protease inhibitors. In these individuals, a regimen consisting of an integrase inhibitor and other drugs with partial activity against drug-resistant virus might be assembled, but how long such regimens can be expected to prevent viral rebound is unclear.
A phase 3 study of fostemsavir, designed to lead to the licensing of the drug for use in treatment-experienced people, randomised participants to receive fostemsavir 600mg twice daily or placebo for 7 days in addition to their failing regimen, after which all participants received fostemsavir for 24 weeks together with a background regimen that had been optimised by resistance testing. Treatment-experienced people were eligible for the randomised study arm if they were unable to assemble a regimen that was likely to be fully suppressive from currently available agents and only had sensitivity to drugs in one or two classes of antiretrovirals.
The study also included an open-label arm for participants without any fully active drug options in the existing classes of antiretroviral agents, who started treatment with fostemsavir and a background regimen optimised by resistance testing.
A total of 272 people were randomised in the ratio 3:1 to the placebo-controlled arm of the study; 99 people were recruited to the open-label arm.
The study population was at high risk of further HIV disease progression and in need of new treatment options. The median CD4 cell count in the placebo-controlled arm was 100 cells/mm3 and 41 cells/mm3 in the open-label arm, 72% of all study participants had a baseline CD4 cell count below 200 cells/mm3 and 41% a CD4 cell count below 50 cells/mm3, indicating a very high risk of AIDS-related illness.
Eighty per cent of study participants had prior exposure to an integrase inhibitor and over 90% were exposed to protease inhibitors. Less than half of participants in the randomised arm had more than one fully active agent in their optimised background regimen (42%). Of the 272 participants randomised, 45 (17%) withdrew by week 24. In the open label arm 26 of 99 participants (26%) withdrew by week 24.
The primary study endpoint was the mean change in viral load (HIV RNA) from day 1 to day 8 in the randomised cohort. Viral load fell by a mean of 0.79 log10 in the fostemsavir group and 0.17 log10 in the placebo group, a difference of 0.625 log10 (p < 0.001). Just under half the fostemsavir group experienced a viral load reduction of > 1 log10 (46%) and 65% experienced a reduction of > 0.5 log10.
Among those with a baseline viral load > 1000 copies/ml (90% of all participants) the median decrease in viral load was 1 log10.
At week 24, 54% in the randomised arm had a viral load below 40 copies/ml, 32% had a viral load above 40 copies/ml and were still taking study medication, 3% had discontinued due to lack of efficacy, adverse events or death, and 6% had changed their optimised background regimen and were classified as virologic failures. The remainder had discontinued treatment due to lack of efficacy, adverse events or death.
Serious adverse events occurred in 30% of participants (including the non-randomised arm) and led to discontinuation of study drug in 6% of all participants. These included AIDS-related infections and other events reflecting the advanced HIV disease of study participants. The most common grade 2-4 drug-related adverse events were nausea, diarrhoea, headache, vomiting, fatigue and weakness (asthenia).
Fostemsavir has been designated a Breakthrough Therapy by the US Food and Drug Administration (FDA) for answering an unmet clinical review and will receive rapid review when submitted for licensing. The FDA is expected to review a licensing application in the 2019 or 2020.
Kozal M (Lataillade presenting). Phase 3 study of fostemsavir in heavily treatment-experienced HIV-1 infected participants: day 8 and week 24 primary efficacy and safety results (BRIGHTE study, formerly 205888/AI438-047). 16th European AIDS Conference, 25-27 October, Milan, abstract PS8/5, 2017.
